-
Introduction
I decided to study telomere telomerase and vascular aging after I came across a review article one day. I don't know if this is what is called chance or fate.
However, I always think, "Nothing happens by chance. I always want to be in control my destiny rather than to just surrender myself to it.” I would like to share with you my past 20 years’ experience. -
Entrance of the basic research
– 3rd Department of Internal Medicine, Faculty of Medicine, the University of TokyoFor five years after graduation, I engaged in clinical training as a doctor of cardiovascular medicine. As with other people, I was attracted to the clinical dynamism of cardiovascular medicine and decided to pursue this path. However, as I gradually became accustomed to the flow of daily clinical practice, I became interested in the pathophysiology of individual diseases. I was particularly impressed with cases of familial dilated cardiomyopathy.
At that time, it was skeptical of the existence of genes which cause this condition. However, I thought that the identification of these genes could cure dilated cardiomyopathy.
Faculty of Medicine, The University of Tokyo
Around that time, I knocked on the door of Dr. Yoshio Yazaki's laboratory, which was a mecca for molecular biology research in the cardiovascular field. He kindly allowed me to participate as a research student. Initially, I was planning to join the heart failure group led by Dr. Issei Komuro (currently Professor of the University of Tokyo), but at the recommendation of Professor Yazaki, I was assigned to the blood vessel group headed by Dr. Yuki Kurihara (currently Professor of the University of Tokyo). There, I decided to conduct research on endothelin and atherosclerosis, a subject which had been actively researched worldwide.
The day I first visited Dr. Kurihara's laboratory, I remember him asking me, "Minamino-kun, where would you like to have your drawers?” At that time, Dr. Yazaki's laboratory did not have much space and about 10 people were frantically doing research from morning till late night in a laboratory about the size of 6-8 tatami mats. I was assigned one set of drawers (I was able to have four sets of drawers over three years) and I started my research. I remember very well that when I could not grab a lab table in the morning, I would do experiment over the lid of the centrifuge.
In the third year, as I was about to finish my bachelor's thesis, I thought of finding a topic that I could work on for the rest of my life. One day, when I went to the library, I noticed an interesting review article on telomerase. It was an article saying that a method was developed in which telomerase activity in human cancer cells could easily be measured. At that time, telomerase had not yet been identified as a molecule, but it was possible to detect it as an enzyme activity. I became very interested in this article and decided to study telomere, telomerase and vascular aging. -
Starting vascular aging research
– Harvard Medical SchoolTelomeres are present at both ends of the chromosome and contribute to its stability. Unfortunately, our DNA replication is not perfect and telomeres become shorter with each division. When telomeres decrease to a certain length, cells recognize the decreased length of telomeres as DNA damage and induce p53-dependent cellular senescence. On the other hand, the enzyme that adds telomeres is telomerase, but since the activity of telomerase is low except for cancer cells and stem cells, telomeres become shorter with every division in normal cells. My hypothesis was that aging at the cellular level causes aging in blood vessels.
Therefore, I thought about studying abroad to start a new research. However, most telomere research at that time was conducted on cancer cells and laboratories specializing in vascular biology did not study the aging of blood vessels. I was unsure as to whether to study blood vessels with the support of a laboratory famous for telomeres or to study telomeres in a laboratory for blood vessels with no support. I chose the latter in order to challenge my ability. To that end, I requested a meeting with Dr. Stella Kourembanas, who ran a laboratory specializing in blood vessels at Harvard University and requested if I could study telomeres as a sub-topic. Although she was conducting research on hypoxia, angiogenesis and vascular remodeling, she immediately agreed. In this way, I was able to start research on "hypoxia and angiogenesis" and "telomeres, telomerase and vascular aging."In spite of living on a very small budget in Boston, it was very enjoyable. Salaries of postdoctoral researchers were low and I was receiving various assistance from the state government, such as egg and milk coupons. I remember being surprised when asked in a questionnaire at the time of receiving the assistance if I had ever eaten anything other than food, such as paper or soil. Since I had no clinical duty at the laboratory, I was able to conduct experiments from morning till midnight. I also returned to the laboratory and conducted experiments on weekends, which elicited criticism from my family. However, in the second year, I began getting results, enabling me to submit papers. However, I received very severe criticism.
Aerial view of the suburbs of Boston where I lived
In a comment from a reviewer, I received a blunt reply such as "Why do we need to study telomeres and telomerase in blood vessels?" By the end of the third year, Japan had entered a prolonged recession after the burst of the bubble economy. At that point, I decided to return to Japan as the company my father ran was affected by the recession.
-
Research on vascular aging after returning to Japan:Graduate School of Medicine, Chiba University
When I returned to Japan, I began working at a hospital affiliated with Chiba University, my alma mater. I was somewhat nervous about conducting catheter treatment for the first time in a few years, but it was enjoyable. As for vascular aging research, I was on the verge of giving up because I could not produce any results after studying abroad for three years. However, after daily observing patients who were urgently hospitalized and receiving angioplasty being hospitalized again because of angina / myocardial infarction caused by another vascular lesion, I felt a strong need that a radical treatment method must be developed.
Around that time, it was decided that the telomere/telomerase and vascular aging paper that was previously not accepted*1 would be accepted. Thus, I decided to resume research on vascular aging. I managed to find time to do research for half a day a week between catheterizations. Thereafter, I persevered and continued to submit papers on my work that I did while studying abroad and was finally able to obtain one publication each for "Hypoxia and angiogenesis" and "Telomere/telomerase and vascular aging." * 2,3After returning to Chiba University, I decided to continue my research on vascular aging and angiogenesis with the permission of Professor Komuro, who had joined the university at that time. By then, research on telomere and cellular aging in blood vessels was starting to be recognized worldwide. Since returning to the university, I could demonstrate that telomere – dependent cellular senescence plays a critical role in vascular aging *4,5, and that telomere – independent cellular senescence (angiotensin and insulin signals) also plays an important role in vascular aging * 6,7. In particular, I was very moved that my paper on heart failure which was accepted by Nature for the first time was a fusion of the two topics (hypoxia and p53) that I was working on while studying abroad *8. I am also very pleased that the data clarifying that telomeres is also related to diabetes *9 could subsequently be publish in Nat Med.
Aerial photograph of the suburbs of Chiba University School of Medicine at that time
-
From Chiba University to Niigata University and
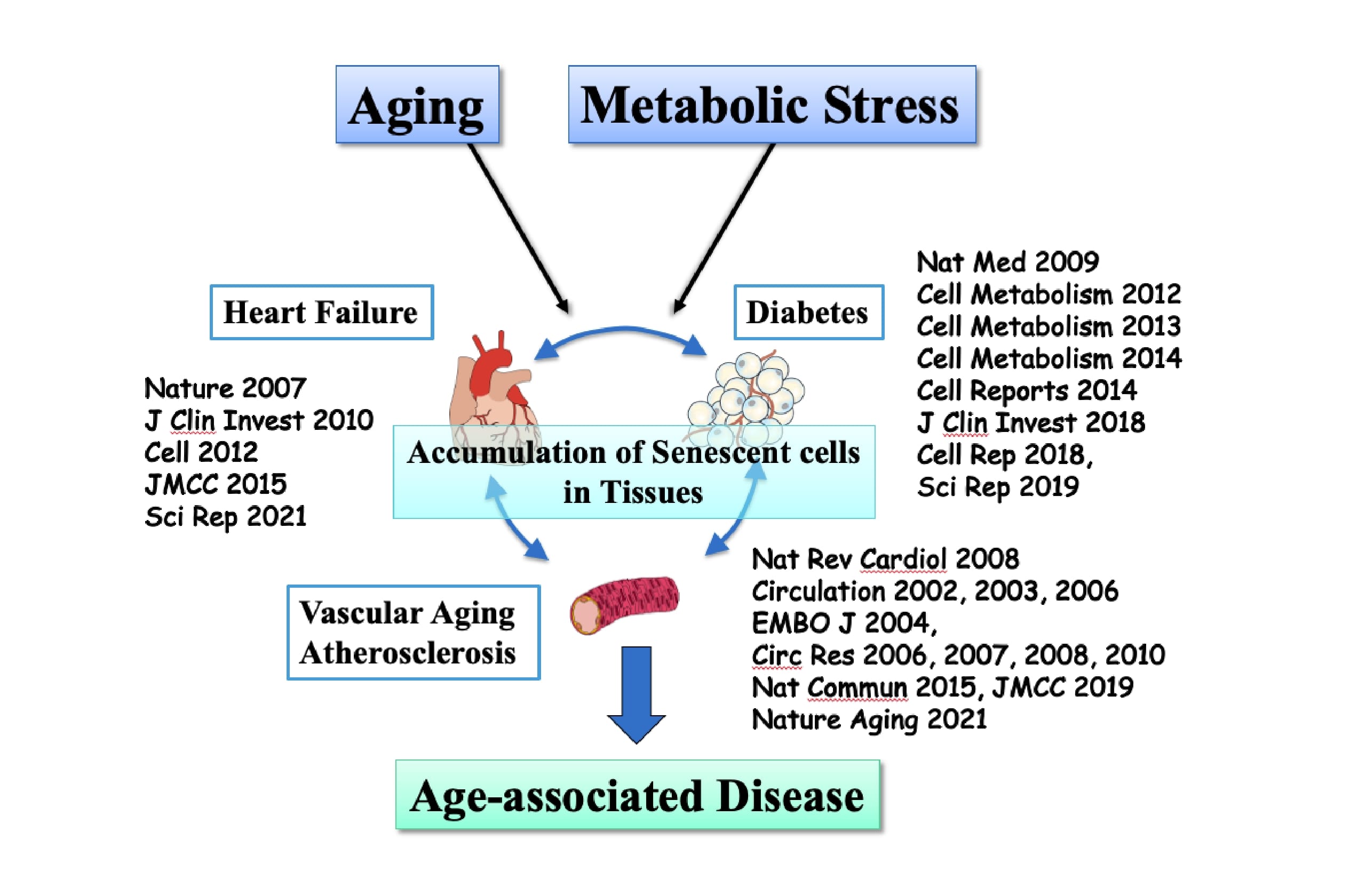
then to Juntendo UniversityI was assigned to preside over the Department of Cardiovascular Biology and Medicine at Niigata University from 2012. It took a considerable amount of time moving research equipment and genetically-modified mice that had been bred in large numbers at Chiba University to Niigata University. However, I was able to further develop "cellular aging research" at Niigata University as well. In Nature and Nat Med where I published my findings during my Chiba University days, I was able to clarify that senescent cells accumulate in the heart and visceral fat (organ aging) due to aging and stress, and the accumulation of senescent cells in the heart and fat causes the onset and progression of heart failure and diabetes, respectively. At Niigata University, I further developed this research, and I could demonstrate that as the heart ages, the visceral fat ages correspondingly, resulting in the deterioration of metabolism and exacerbation of heart failure *10. Also, the molecule called semaphorin is the key molecule that links fat aging to diabetes *11. Moreover, as blood vessels age, the risk of developing diabetes increases *12.
Furthermore, I was given the opportunity this time to play an active role in the Department of Cardiovascular Biology and Medicine at Juntendo University. I have further advanced these research work and proved that removing accumulated senescent cells (Senolysis) would increase healthy life expectancy. I would like to take this opportunity to say that thanks to many people, I have been able to continue my research thus far and I will strive to do my best to conduct an even more meaningful research in the future.
-
Conclusion
There are innumerable fields of medical research, both basic and clinical. However, I feel that it is important to focus on one field and improve oneself in that path. Moreover, I think it's important to take advantage of chance encounter and not to succumb to misfortune.
When I returned to Japan without receiving any recognition for the research work that I started while studying abroad in Boston, I temporarily lost my passion for medical research and medical care. However, after resuming work at the clinical departments of related hospitals in the region and experiencing the reality that "stent treatment does not improve the prognosis of coronary artery disease," I was able to regain my passion of developing a treatment for radical cardiovascular diseases.
In the medical field in Japan at present, I feel that an increasing number of doctors are losing their passion for medical research and medical care due to weak interpersonal relationships coupled with economic and social circumstances.
Therefore, by offering comprehensive education and advanced medical care, and conducting innovative research, I would like to create an appealing cardiology class which will inspire many students, residents, and senior doctors to take the initiative to enroll. I would also like to nurture doctors who are able to identify their path and have a strong passion for it, whether it be community-based healthcare, advanced medical care or basic research, etc. -
References
- Minamino, T., Mitsialis, S. A. & Kourembanas, S. Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol Cell Biol 21, 3336-3342 (2001).
- Minamino, T. et al. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci U S A 98, 8798-8803 (2001).
- Minamino, T. & Kourembanas, S. Mechanisms of telomerase induction during vascular smooth muscle cell proliferation. Circ Res 89, 237-243 (2001).
- Minamino, T. et al. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105, 1541-1544 (2002).
- Minamino, T. & Komuro, I. Vascular aging: insights from studies on cellular senescence, stem cell aging, and progeroid syndromes. Nat Clin Pract Cardiovasc Med 5, 637-648 (2008).
- Kunieda, T. et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation 114, 953-960 (2006).
- Miyauchi, H. et al. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. Embo J 23, 212-220 (2004).
- Sano, M. et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446, 444-448 (2007).
- Minamino, T. et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15, 1082-1087 (2009).
- Shimizu, I. et al. p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab 15, 51-64 (2012).
- Shimizu, I. et al. Semaphorin3E-induced inflammation contributes to insulin resistance in dietary obesity. Cell Metab 18, 491-504 (2013).
- Yokoyama, M. et al. Inhibition of endothelial p53 improves metabolic abnormalities related to dietary obesity. Cell Rep 7, 1691-1703 (2014).
StoryTohru Minamino ‐ steps・career
Story